Clinical SAS
- 100% Placement Assistance
- Industry-Ready Curriculum
- Hand-on Practical Experience
A diploma course for pharma & IT professionals
In Collaboration With :

Live Online Classes starting on 8th, 15th, 22nd & 30th.
Book Your Free Demo Class Today!

IAO & NSDC Accredited Courses

100% Placement Assistance

Hands-on Practical Training

Industry-led Expert Trainers
Our Courses

Certification in Clinical SAS
Demonstrate your professional ability to use SAS
programming software for clinical trial programming
with globally recognized certification.

Advanced Diploma in Clinical SAS
Learn how to use SAS Software for clinical trials, and
gain expertise in data analysis and clinical SAS with
advanced diploma in clinical SAS
Career Paths after Clinical SAS Diploma Courses
Clinical Research Associate
Highlight the relevance of clinical research after BDS while understanding trials procedures and interacting with dental professionals in clinical trials.
Responsibilities: Monitoring clinical trials, ensuring adherence to protocols, data collection, and interacting with investigators.
Clinical Data Analyst (CDA)
Manage information, identify data trends, maintain systems, ensure high-quality and safe data following healthcare informatics regulations.
Responsibilities:
Collect and record data, Analyze data trends,
Create reports, Design systems
Clinical SAS Programmer
Use statistical and mathematical methods to analyze and interpret clinical trial data.
Responsibilities: Develop SAS macros, Templates and utilities for
data cleaning and reporting
Regulatory Affairs
Responsibilities: Ensuring compliance with regulatory guidelines (e.g., GCP, ICH-GCP).
Pharmacovigilance
Responsibilities:Monitoring drug safety and adverse events.
Career Paths after Clinical Research Diploma Courses
Clinical Research Associate
Highlight the relevance of clinical research after BDS while understanding trials procedures and interacting with dental professionals in clinical trials.
Responsibilities: Monitoring clinical trials, ensuring adherence to protocols, data collection, and interacting with investigators.
Clinical Data Manager (CDM)
Emphasize the importance of attention to detail and organizational skills, valuable assets for clinical research after BDS.
Responsibilities:Data cleaning, validation, and analysis, ensuring data accuracy and integrity.
Clinical Research Coordinator (CRC)
Highlight the patient interaction and communication skills developed during BDS studies.
Responsibilities:Assisting investigators in conducting clinical trials, managing study logistics, and interacting with patients.
Regulatory Affairs
Responsibilities: Ensuring compliance with regulatory guidelines (e.g., GCP, ICH-GCP).
Pharmacovigilance
Responsibilities:Monitoring drug safety and adverse events.
What Sets CliniLaunch Apart

Placement Assistance
Strong network of recruiters from the healthcare, pharmaceutical, and biotechnology industries and offer placement assistance to students.
Industry Expert Trainers
Equip yourself with skills and knowledge under the mentorship of experienced faculties with over 17 years of experience in the field of healthcare research and training.
Learning Management System
Embark on a transformative learning experience with our state-of-the-art Learning Management System!
Non-Technical and Technical Sessions
Go beyond the textbook with a well-rounded foundation balancing essential technical and non-technical skills needed to thrive in healthcare, IT and Pharma.
Job Oriented Programs
Get comprehensive job-oriented programs to empower you with the skills and knowledge you need to succeed in the dynamic and competitive healthcare sector.
Clinical SAS Training Online course syllabus
Curriculum Designed by Experts
Common curriculum for Clinical SAS classes online
- SAS Coding Fundamentals
- Data Management and Integrity
- CDISC Standards
- Statistical Reporting in Clinical Trials
- Data Visualization with SAS
- Advanced Analytics Techniques
- Regulatory Compliance and Submission
FREE Career Counselling
We are happy to help you 24/7
A Student’s Journey



Industry-Ready Training
Equip yourself with skills and knowledge required to be successful in the healthcare-pharma or healthcare-IT industry. Enhance your communication and personality.
Certified Courses
Earn credentials through online or in-person programs validating the enhancement of your skills and expertise in the healthcare-IT and pharma sector.
Get Placed
Gain access to volunteer, internship, and placement opportunities and apply real-world applications in healthcare settings like hospitals, CROs, and pharma companies.
FAQs – Clinical SAS Programming Course Online
A clinical SAS programming course focuses on a specific set of skills essential for working with clinical trial data. The core programming skills include Base SAS for fundamental data manipulation and reporting, and Advanced SAS for more complex tasks like macro programming for automation and PROC SQL for data querying. You will learn to write code to clean, validate, and analyze raw data, as well as generate professional reports such as tables, listings, and figures (TLFs). The course ensures you are proficient in writing efficient and reliable code for the specific demands of the clinical research industry.
A clinical SAS programming course focuses on a specific set of skills essential for working with clinical trial data. The core programming skills include Base SAS for fundamental data manipulation and reporting, and Advanced SAS for more complex tasks like macro programming for automation and PROC SQL for data querying. You will learn to write code to clean, validate, and analyze raw data, as well as generate professional reports such as tables, listings, and figures (TLFs). The course ensures you are proficient in writing efficient and reliable code for the specific demands of the clinical research industry.
The curriculum is structured to guide you from foundational concepts to advanced specialized topics. It begins with an introduction to the SAS environment and basic data steps. As you progress, you will delve into more complex data manipulation, statistical procedures, and reporting tools. A significant portion of the course is dedicated to the application of these skills to CDISC standards (SDTM and ADaM), which are the global requirements for clinical data submission to regulatory bodies.
Projects in a Clinical SAS Programming Course are designed to simulate real-world scenarios. You will work with sample datasets from clinical trials to perform tasks such as creating standardized datasets, generating demographic summaries, and reporting on adverse events. These projects are crucial because they provide hands-on experience and allow you to build a portfolio of work that demonstrates your practical skills to potential employers.
While both teach the SAS language, a Clinical SAS programming course from Clini Launch is highly specialized. A general course focuses on broader applications in various industries like finance or retail. In contrast, a clinical course is entirely centered on the unique data structures, regulatory requirements (like CDISC), and reporting needs of the pharmaceutical and clinical research industries. The entire curriculum is tailored to prepare you for a specific career path as a Clinical SAS Programmer.
No, prior programming knowledge is not strictly required for a Clinical SAS programming course. Many Clinical SAS programming courses from Clini Launch are designed for beginners and start with an introduction to the SAS programming language from scratch. A background in a related field like life science, pharmacy, or statistics can be beneficial, but the course will provide all the necessary technical training to become a proficient programmer.
The main purpose of a Clinical SAS Certification is to formally validate your skills and knowledge to employers. Issued by the SAS institute, the certification is an industry-recognized credential that proves you have the expertise required to program clinical trials and adhere to regulatory standards. It provides a significant advantage in the job market, as it demonstrates a high level of proficiency and commitment to the field.
The official Clinical SAS Certification exam, also known as the SAS Certified Clinical Trials Programmer, is a time-bound test with a mix of multiple-choice and short-answer questions. It assesses your practical skills in using SAS for clinical trial data. Topics covered include the clinical trials process, CDISC data structures (SDTM and ADaM), data transformation, and reporting. You must have a strong foundation in Base and Advanced SAS to pass the exam.
Holding a Clinical SAS Certification can significantly boost your career. It can lead to higher starting salaries, better job opportunities, and faster career progression. Many companies, especially large pharmaceutical firms and CROs, prefer or even require candidates to be certified. It also gives you a competitive edge over uncertified applicants and can help your secure promotions into senior or lead programmer roles.
To prepare, a candidate should first complete a comprehensive Clinical SAS Certification course from Clini Launch is aligned with the official exam curriculum. Beyond the course, it is essential to practice extensively with hands-on projects and mock exams. Studying the official SAS exam content guide is also crucial, as it provides a detailed list of all the topics and skills that will be tested.
The SAS institute’s main certification for this field is the SAS Certified Clinical Trials Programmer Using SAS 9. While this is the most common one, many professionals also pursue the SAS Certified Base Programmer and Advanced Programmer certifications as a prerequisite or in parallel. These certifications build a strong foundation and demonstrate a comprehensive skill set.
A Clinical SAS Institute serves as a vital bridge between aspiring professionals and the clinical research industry. It provides structured training, expert guidance, and industry-specific knowledge that is difficult to acquire through self-study alone. Clini Launch teaches you technical skills and also helps you understand the clinical trial process, regulatory requirements, and the specific demands of a professional role.
When choosing a Clinical SAS institute, you should consider several factors to ensure a quality learning experience. First, check the instructors’ qualifications and industry experience. Second, evaluate the curriculum to ensure it covers all essential topics, including CDISC standards and real-time projects. Third, look for institutes with a strong reputation for placement assistance and positive reviews from past students.
Clini Launch provides hands-on experience through a combination of methods including access to the SAS software and providing you with datasets from simulated clinical trials. You will work on a series of projects and assignments, such as cleaning data, deriving new variables, and generating reports. This practical, project-based learning is the cornerstone of a good training program.
A good Clinical SAS Institute provides comprehensive support that extends beyond the classroom. This includes resume building workshops, mock interviews, and career counselling to help you prepare for the job market. Clini Launch have dedicated placement teams that work with a network of pharmaceutical companies and Contract Research Organizations (CROs) to help you secure a job after course completion.
The average duration of a course from a Clinical SAS Institute can vary. A basic course might take 2-3 months, while a more comprehensive program that includes avdnaced topics and extensive project work can last anywhere from 4-6 months. The length of the program often corresponds to the depth of the curriculum and the level of expertise you will achieve.
The curriculum of a Clinical SAS Course is meticulously designed to be highly practical. It focuses on the specific data formats and challenges encountered in clinical trials. For example, you will learn to work with datasets containing patient demographics, adverse events, and lab results. The course is built around CDISC standards so that every programming technique you learn is directly applicable to a professional role and produces outputs that meet regulatory guidelines.
A student will gain a blend of technical and domain-specific skills. Technical skills include SAS programming, data manipulation, and report generation. The domain-specific skills are equally important and include an understanding of the clinical trial lifecycle, Good Clinical Practice (GCP) principles, and the purpose of different types of clinical data. These combined skills make you a valuable asset to an employer.
Yes, Clini Launch’s Clinical SAS Course providers include career services as part of their training package. These services can be crucial for landing a job. Clini Launch include mock interviews to help you practice answering common questions, resume-building workshops to highlight your new skills, and individual counseling to help you navigate your job search and professional goals.
The instructor’s background is very important at Clini Launch. A quality Clinical SAS Course is taught by an instructor who has extensive experience working as a programmer in the clinical research industry. Their real-world knowledge is invaluable and ensures that the lessons you learn are relevant and up to date with current industry practices. They can also share practical insights that you won’t find in a textbook.
Clini Launch’s Clinical SAS Course can be found in several learning environments. It can be a traditional in-person class, a live online class with a scheduled time, or a self-paced online course where you learn at your own speed using pre-recorded videos. Each format offers different levels of flexibility and interactivity, so you can choose the one that best suits your learning style.
A comprehensive Clinical SAS Training program from Clini Launch has three main components. First, it provides a deep dive into the SAS programming language, including both foundational and advanced topics. Second, it educates you on the clinical research domain and regulatory standards like CDISC. Third, it offers extensive hands-on experience through projects and case studies that simulate real-world clinical trial data analysis. All three components are essential for becoming a proficient programmer.
Clinical SAS Training program from Clini Launch places a strong emphasis on data quality and validation, as these are critical to the integrity of clinical trials. You will learn to write code to check for inconsistencies, missing values, and logical errors in a dataset. You will also learn about the principles of programming validation, which involves creating a second program to verify the results of the first program. This rigorous process is a key part of the training.
A good Clinical SAS Training program includes a variety of projects that cover the full lifecycle of a clinical trial. Clini Launch’s Clinical SAS Training program includes projects on data cleaning and manipulation, creating standardized SDTM datasets, and generating ADaM analysis datasets. You will also work on producing final reports like efficacy and safety tables. These projects help you build a portfolio of work that demonstrates your ability to handle different tasks.
Yes, there is a consistent demand for professionals with Clinical SAS Training. As the number of clinical trials grows, so does the need for skilled programmers who can manage and analyze the data. Pharmaceutical companies and CROs are constantly looking for trained individuals who can ensure that clinical trial data is processed accurately and efficiently for regulatory submission.
The field of clinical research is always evolving, so it’s important to stay current. Professionals can do this by keeping up with changes in CDISC standards, participating in professional forums, and pursuing additional certifications. Many organizations also offer advanced training courses on new technologies or programming techniques to help professionals stay at the forefront of the industry.
The main benefit of a Clinical SAS Programming Online Course from Clini Launch is its flexibility. It allows you to learn from anywhere in the world and at a pace that fits your schedule. This is particularly beneficial for those who are currently employed or have other commitments. The online format removes geographical barriers and provides access to high-quality instructors and materials that may not be available locally.
A quality Clini Launch’s Clinical SAS Programming Online Course provides you with access to a virtual SAS environment where you can practice coding and work on projects. You will be provided with datasets to simulate real-world scenarios, and your work will be reviewed by instructors. This approach ensures that you gain the same practical, hands-on experience as a traditional in-person course, but with the added convenience of an online format.
You can expect a variety of support options. Clini Launch’s Clinical SAS programming online course offers live sessions where you can ask questions directly to instructors, as well as dedicated discussion forums for interacting with peers. Industry instructors from Clini Launch also provide one-on-one sessions for personalized guidance. This support system is designed to help you overcome any challenges you may face while learning and to keep you on track.
A Clinical SAS Programming online course is an excellent tool to build your portfolio, and these projects can be saved and compiled into a professional portfolio. You can showcase your ability to handle different types of clinical data and demonstrate your mastery of various programming techniques.
A structured online course is more effective than self-study. A Clinical SAS Programming online course from Clini Launch provides a clear, guided path from beginner to advanced topics. It includes expertly curated materials, hands-on projects, and instructor support that a self-study approach lacks. The structure and accountability of a course increases your chances of successfully learning the material and preparing for a career.
Clinical SAS training and placement from Clini Launch refers to a comprehensive program that not only teaches you the skills to become a Clinical SAS programmer but also provides dedicated support to help you secure a job in the industry. It’s an all-in-one solution that takes you from a beginner to a job-ready professional. The training component focuses on skills, while the placement component focuses on career opportunities.
Placement assistance from Clini Launch includes various services. This often includes resume and cover letter writing workshops, mock interview practice, and networking opportunities with recruiters and industry professionals. The institute may also have a dedicated placement team that works with a network of pharmaceutical companies and CROs to find suitable job openings and refer qualified candidates.
The career prospects are very strong. Graduates from Clini Launch can work in roles such as Clinical SAS Programmer, Statistical Programmer, or Clinical Data Analyst. These jobs are available in pharmaceutical companies, biotech firms, and contract research organizations. The demand for these roles is consistently high, and the starting salaries are often competitive.
The typical starting salary for a fresher who has completed a Clinical SAS training and placement program from Clini Launch can range from ₹3.5 to ₹6 lakhs per annum. This can vary based on the company, the location, and the specific skills a candidate possesses. Experience and additional certifications can lead to a significant increase in salary over time.
The main advantage of a Clinical SAS course online is its unparalleled flexibility. It allows you to study at your own pace and on your own schedule, which is ideal if you have a full-time job or other commitments. You can access course materials, lectures, and assignments from anywhere in the world, making it a highly accessible option for a wide range of learners.
Practical exercises are handled through virtual SAS environments or cloud-based platforms. A quality Clinical SAS course online from Clini Launch will provide you with access to the SAS software, allowing you to run your code and complete hands-on projects. You will be given datasets to work with, simulating the real-world data you would encounter in a professional setting. This ensures you gain essential practical experience remotely.
The support for a Clinical SAS Course online is quite comprehensive. It offers live, instructor-led sessions, dedicated Q&A forums, and email support. Clinical SAS Courses from Clini Launch also provide one-on-one mentorship. This ensures that even though you are not in a physical classroom, you have multiple channels for getting help and staying engaged with the course material.
A student can build a portfolio by saving all the projects they complete during the Clinical SAS course online. These projects, which often include tasks like creating standardized datasets and generating reports, can be compiled and showcased. A strong portfolio, which demonstrates a candidate’s practical skills, is often more important to employers than the mode of learning.
Yes, Clini Launch’s Clinical SAS course online programs are regularly updated to reflect the latest industry trends. They incorporate new technologies like SAS Viya, discuss the use of AI in clinical programming, and keep the curriculum aligned with the most recent CDISC standards. This ensures that the skills you learn are current and relevant to the needs of the industry.
CliniLaunch recent placed students
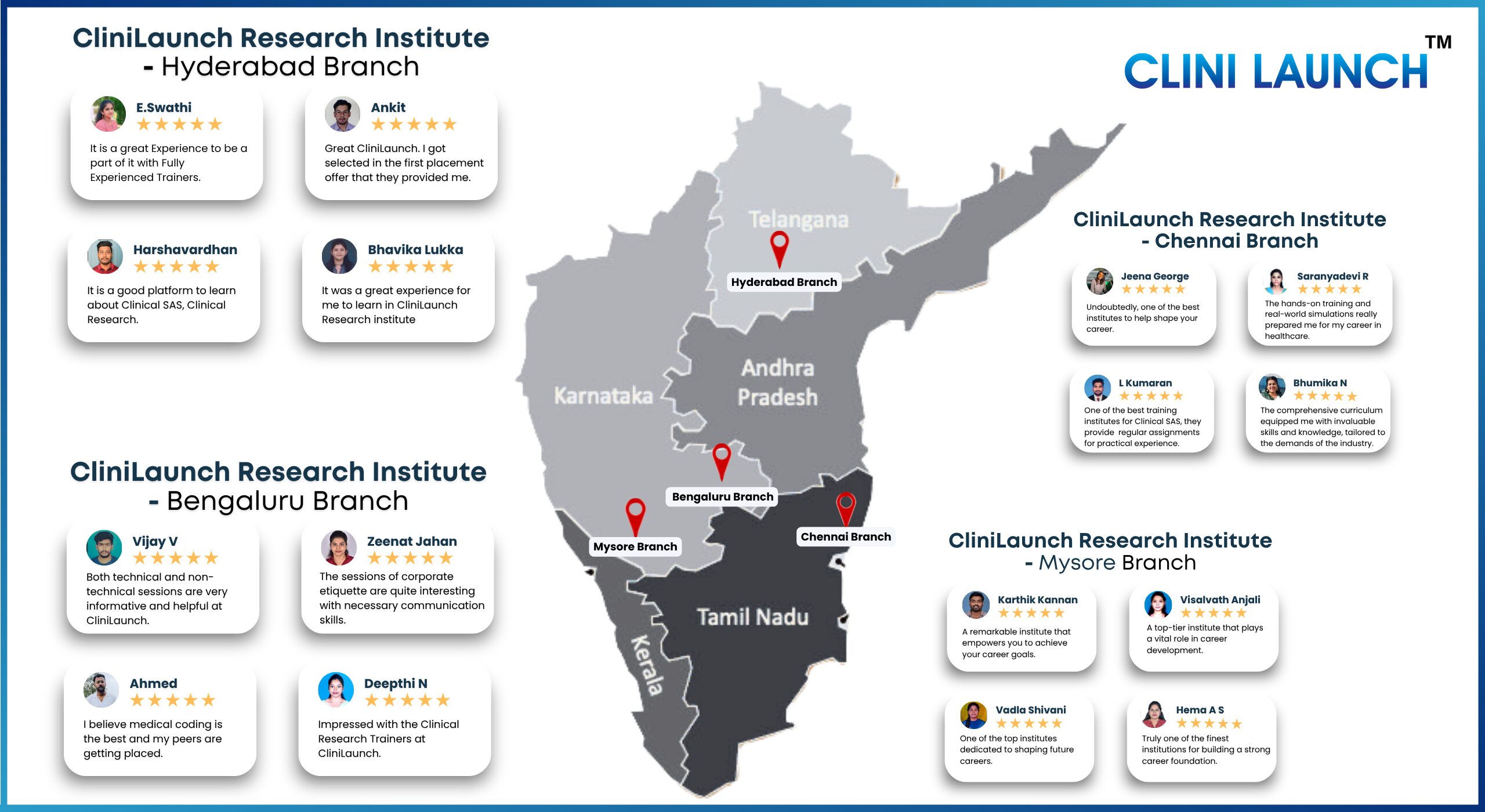


















Testimonials
Upskilling does make a difference. Graduates speak out.Hear what our students and professionals are saying about their upskilling journey with CliniLaunch.
Posted on

