Clinical SAS Course in Kerala
Clinical SAS course in Kerala is your gateway to a rewarding career in the healthcare, pharmaceutical, and biotechnology industries. By enrolling in the program from Clini Launch, you will be able to use SAS (Statistical Analysis System) software for specific tasks for analyzing, managing, and reporting clinical trial data. We offer:
- 100% Placement Assistance
- Hands-on Practical Learning
- Industry-led Expert Trainers
- Globally Recognized Certificate
Prepare yourself for the role of Clinical Data Manager for Pharmaceutical or Healthcare companies.
Book Your Free Demo Class Today!
Clinical SAS Course in Kerala
Clinical SAS course in Kerala is your gateway to a rewarding career in the healthcare, pharmaceutical, and biotechnology industries. By enrolling in the program from Clini Launch, you will be able to use SAS (Statistical Analysis System) software for specific tasks for analyzing, managing, and reporting clinical trial data. We offer:
- 100% Placement Assistance
- Hands-on Practical Learning
- Industry-led Expert Trainers
- Globally Recognized Certificate
Prepare yourself for the role of Clinical Data Manager for Pharmaceutical or Healthcare companies.
Book Your Free Demo Class Today!

IAO & IAF Accredited Course

LSSSDC Membership

Practical Industry Exposure

Industry-led Expert Trainers
Our Courses

Certification in Clinical SAS
Learn to transform and handle clinical trial data while utilizing SAS software and to cultivate expertise in generating reports and visual representation for clinical investigators.

Advanced Diploma in Clinical SAS
Focus on building the skills required to manage and submit CDISC standard reports using clinical programming, data validation techniques, and regulatory submissions using real-world scenarios.
Career Paths after Clinical SAS Course in Kerala

Clinical SAS Programmer
As a Clinical SAS programmer, you will utilize SAS software to develop and refine codes for various tasks within clinical trials along with data handling, statistical examination, and report documentation.
Key Responsibilities: Craft SAS macros, templates, and tools for data refinement and reporting processes.
Clinical Research Associate
Clinical Research Associate closely works with clinical data managers, and biotechnicians, employing SAS software to oversee data and report on trial advancement.
Key Responsibilities: Supervise clinical trials, ensure compliance with study guidelines, manage data collection, and liaise with researchers.


Pharmacovigilance Analyst
Pharmacovigilance analysts specifically there to track safety and effectiveness in products after the drugs introduced in the market.
Key Responsibilities: Examine post-market data, pinpoint potential hazards, and submit reports to regulatory bodies.
Biostatistician
Biostatisticians specifically leverage SAS software tools and techniques applying statistical concepts and methodologies to analyze clinical trial data concerning the efficacy and safety of medication or treatments.
Key Responsibilities: Design research studies, interpret findings, and communicate results effectively.

Clinical Data Analyst
Clinical data analysts work intimately with SAS software to scrutinize patterns, tendencies, and possible problems within the data, delivering valuable insights for research and development initiatives.
Key Responsibilities: Gather and document data, investigate data patterns, produce reports, and develop systems.
Career Paths after Clinical SAS Course in Kerala
Clinical SAS Programmer
As a Clinical SAS programmer, you will utilize SAS software to develop and refine codes for various tasks within clinical trials along with data handling, statistical examination, and report documentation.
Key Responsibilities: Craft SAS macros, templates, and tools for data refinement and reporting processes.
Clinical Research Associate
Clinical Research Associate closely works with clinical data managers, and biotechnicians, employing SAS software to oversee data and report on trial advancement.
Key Responsibilities: Supervise clinical trials, ensure compliance with study guidelines, manage data collection, and liaise with researchers.
Pharmacovigilance Analyst
Pharmacovigilance analysts specifically there to track safety and effectiveness in products after the drugs introduced in the market.
Key Responsibilities: Examine post-market data, pinpoint potential hazards, and submit reports to regulatory bodies.
Biostatistician
Biostatisticians specifically leverage SAS software tools and techniques applying statistical concepts and methodologies to analyze clinical trial data concerning the efficacy and safety of medication or treatments.
Key Responsibilities: Design research studies, interpret findings, and communicate results effectively.
Clinical Data Analyst
Clinical data analysts work intimately with SAS software to scrutinize patterns, tendencies, and possible problems within the data, delivering valuable insights for research and development initiatives.
Key Responsibilities: Gather and document data, investigate data patterns, produce reports, and develop systems.
Eligibility Criteria for Clinical SAS Course in Kerala
The eligibility criteria for Clinical SAS course in Kerala generally requires a bachelor’s or master’s degree. We at Clini Launch prefer graduates from Life sciences, statistics, mathematics or computer science backgrounds. Our course requires you to have an understanding of statistics or programming as it is beneficial for students with little to no-prior programming experience while emphasizing a strong logical and analytical attitude.
Designations


Want to become a Clinical Data Analyst?
What Sets CliniLaunch Apart

Dedicated Placement Support
Benefit from our robust connections with hiring managers across the healthcare, pharmaceutical, and biotech sectors, providing you with comprehensive placement assistance.
Experienced Industry Mentors
Gain invaluable skills and insights from seasoned instructors boasting over 17 years of expertise in healthcare research and education.
Advanced Learning Platform
Immerse yourself in a revolutionary educational journey through our cutting-edge Learning Management System!
Comprehensive Skill Development
Develop a holistic understanding with our balanced curriculum, encompassing vital technical and non-technical proficiencies essential for success in healthcare, IT, and Pharma.
Career-Focused Programs
Access thorough, job-centric programs designed to equip you with the necessary skills and knowledge to excel in the dynamic healthcare industry.
Clinical SAS Course Curriculum
Core syntax | Programming Concepts of SAS including Data Step Processing | PROC steps (like PROC PRINT, PROC SORT, PROC MEANS), data manipulation (e.g., merging, appending, datasets), and creating basic reports. It forms the bedrock for all subsequent analytical work.
This module focuses on quality, accuracy, and reliability of clinical trial data covering data validation, cleaning, error identification, handling missing values, and implementing robust data integrity checks. It is just to ensure the data is suitable for analysis and regulatory submission.
This is a crucial module that delves into the clinical data interchange standards Consortium (CDISC) guidelines, which are globally mandated for clinical trial data submission to regulatory authorities like Foods and Drug Administration. These modules let you cover SDTM (Study Data Tabulation Model) for organizing raw data and ADaM (Analysis Data Model) for creating analysis datasets, ensuring data traceability and consistency.
Statistical reporting in clinical trials teaches you how to generate various statistical reports in clinical trials. This may include creating tables, listings, and figures (TLFs) for study results, adverse events, patient demographics, and efficiency/safety endpoints by using appropriate SAS procedures for statistical analysis (e.g., PROC TTEST, PROC ANOVA, and PROC FREQ).
Data visualization with SAS focuses on creating compelling and informative visual representation of clinical data using SAS graphical procedures (e.g., PROC SGPLOT, PROC GCHART). Participants design effective charts, plots, and dashboards to communicate valuable insights from clinical trials clearly and concisely.
Going beyond basics of statistics, this module introduces more sophisticated analytical methods applicable to clinical data. This might include survival analysis, logistic regression, mixed models, or other advanced statistical techniques for in-depth exploration of data relationships and prediction.
Regulatory compliance and submission provide a critical understanding of the regulatory bodies for clinical trials while adhering to guidelines from agencies like the FDA and EMA. It covers the process of preparing and submitting electronic clinical trial data packages (e.g., eCTD) in compliance with regulatory requirements, ensuring smooth review and approval processes.
FREE Career Counselling
We are happy to help you 24/7
A Student’s Journey



Industry-Ready Training
Learn and equip yourself with skills required to be successful in healthcare, pharma, & IT industry. Enhance your communication and personality.
Certified Courses
Earn credentials through online or in-person programs validating the enhancements of your skills and expertise in the healthcare, IT, and Pharma sectors.
Get Placed
Gain access to live training sessions, industry guest speakers, internships, volunteering, and placement opportunities. Apply it in real-world applications.
Level up your Career with Clinical SAS Course in Kerala
Real people. Real results.
Master in-demand skills and knowledge
Propel your career in healthcare and pharma industry with CliniLaunch.

Unlock your potential Mentorship advantage
Personalized industry experts with assistance for your career choices.

Navigate your medical career with confidence
Unlock your potential with upskilling in what you truly enjoy.

Why Clini Launch?
Industry Driven Curriculum
- Adapt to industry standards
- Gain in-depth knowledge
- Up to date your skill sets
Experienced Instructors
- Interact with seasoned professionals
- Insightful healthcare practices
- Dedicated industry experts
Hands-on Learning
- Live interactive session
- Integrated case studies
- Practical step guidance
Industry Recognized Accreditations
- International Accreditation Organization (IAO)
- International Accreditation Forum (IAF)
- National Skill Development Council (NSDC)
Career Support Services
- Access to Career support group
- Resume Building Workshops
- Placement Mentorship Program
FAQs for Clinical SAS Course in Kerala
Once your complete the course from Clini Launch’s Clinical SAS course in Kerala, freshers can look for career prospects as Clinical Data Analyst, SAS Clinical Programmer, Biostatician, Clinical Data Manager, and others. Freshers should always look for Clinical research organizations (CROs), pharmaceutical companies, and healthcare IT firms. However, as the demand for such roles is growing with the expansion of the life science and relevant sectors.
A comprehensive clinical SAS course in Kerala usually spans around 3 to 6 months for certification in Clinical SAS and Advanced Diploma in Clinical SAS. The fees may vary depends on the modules you choose, and the depth of the curriculum along with placement assistance at Clini Launch.
While there are no specific prerequisites, it may vary based on the course you choose as Clini Launch offer two courses including Certification and Advanced Diploma in Clinical SAS. These are basic courses requires a bachelor’s or master’s degree in Life science (e.g., Biotechnology, Biochemistry, Microbiology, Pharmacy), Mathematics, Statistics, or Computer Science is preferred. Clini Launch considers MBBS and BDS with a strong aptitude for programming.
Clini Launch’s Clinical SAS Course in Kerala offers opportunities for internships and industry exposure after the course completion. This may involve you to work on a simulated clinical trial dataset, capstone projects, to mimic industry scenarios, or in some cases, our trainers provide guidance for securing short-exam internships with local CROs (Contract Research Organizations) or pharma companies to gain practical experience.
The most widely recognized SAS Clinical Trials Programmer Certification is the SAS certified Trials Programmer using SAS 9. The courses from Clini Launch – Certification in Clinical SAS and Advanced Diploma in Clinical SAS program offers specialized preparation for this certification. It validates your professional expertise in using SAS to manage, analyse, and report clinical trial data demonstrating proficiency in Base SAS, SAS Macro language, SQL, and critical adherence to CDISC standards (SDTM, ADaM) for regulatory submissions.
The estimates cost for the official SAS clinical trials Programmer certification exam, for example, Exam A00-280 or A00-282 for SAS 9.4 is approximately 15K Indian Rupees. SAS certifications generally do not have an expiration date. They are considered valid indefinitely unless a significant version upgrade of SAS or industry standards renders the certified skills largely obsolete, though periodic updates or new certifications might be recommended.
The recommended preparation strategy for the SAS clinical trials programmer certification includes:
- Mastery of Base and Advanced SAS programming fundamentals.
- Thorough understanding and practical application of CDISC SDTM and ADaM standards.
- Familiarity with clinical trial processes and regulatory requirements (ICH-GCP, FDA).
- Extensive hands-on practice with clinical datasets, creating TLFs.
- Utilizing official SAS study guides, practice exams, and relevant online resources.
By holding a SAS Clinical Trials Programmer Certification, it significantly impacts your career growth and salary potential by:
- Enhancing credibility and marketability in the highly competitive pharmaceutical and research industries.
- Validating a specialized skill set that is in high demand on a global level.
- Leading to higher starting salaries with faster career progression in comparison to non-certified professionals.
- Opening doors to more senior roles like Lead programmer or statistical programming manager.
It is very challenging to provide an exact universal figure, Clini Launch’s Clinical SAS training and placement offers 100% job assistance. However, it typically depends on the students’ aptitude, interview performance, and the institute’s industry connections. It is advisable to check recent placement records and testimonials.
Clinical SAS training and placement program typically offers support beyond just arranging job interviews, including:
- Resume building and optimization specific to clinical SAS roles.
- Mock interview sessions with personalized feedback.
- Soft skill training (communication, professionalism).
- Guidance on aptitude tests and technical assessments.
- Post-placement support or mentorship in initial job phases.
- Guidance on aptitude tests and technical assessments.
- Post placement support or mentorship in initial job phases.
- Networking opportunities with industry professionals.
Clini Launch’s Clinical SAS Training and Placement models connect students with potential employers though:
- Placement Assistance: Clini Launch’s dedicated placement cell maintains relationships with pharmaceutical companies and healthcare IT firms.
- Career Fairs: At Clini Launch, we provide campus recruitment drives where companies directly visit the institute.
- Referral Program: We drive referral programs through alumni network.
- Industry Events: This is especially for clinical research professionals with SAS software skills to get the latest industry insights for their career growth and expansion.
When evaluating the effectiveness of a Clinical SAS training and placement at Clini Launch, we consider:
- Transparency in placement records like number of students placed in the companies and their roles & responsibilities.
- Quality of companies like reputable pharma or contract research organizations that recruit from the Clini launch.
- Types of roles offered to freshers like entry-level programmer vs. data entry.
- Alumni Testimonials and their current career paths for their prospects.
- Dedicated placement team and services they provide like mock interviews, resume preparation, and other relevant services.
- The average time it takes for students to get placed after they complete the course.
The main benefits of choosing a clinical SAS course online for flexibility and accessibility are:
- Learn from anywhere: This is to eliminate geographical barriers, allowing students to access top-tier training regardless of their location.
- Flexible scheduling: By gaining the flexible schedule options, Clini Launch offers recorded sessions, and often weekend or evening live classes to accommodate working professionals or students.
- Reduction in commuting time and costs that helps you save on travel expenses and time, making it more convenient and economical.
- With the access to diverse instructors, you will have the opportunities to learn from experienced professionals globally.
A Clinical SAS course Online ensures adequate hands-on practice by:
- Providing remote access to cloud-based SAS environments (e.g., SAS Studio, SAS Viya).
- Guiding students to install and utilize SAS University Edition for self-practice.
- Integrating a large number of practical exercises, assignments, and capstone projects using real or simulated clinical trial datasets.
- Offering opportunities for code review and debugging support from instructors.
Common technical support and interactive features in a Clinical SAS course Online include:
- Dedicated technical support for software installation and troubleshooting.
- Live Q&A sessions during online classes.
- Online discussion forums or dedicated chat groups (e.g., WhatsApp, Slack) for peer-to-peer and instructor interaction.
- Screen-sharing capabilities for instructors to assist with coding issues.
- Regular assessments with automated and personalized feedback.
Yes, the certification obtained from a Clini Launch’s clinical SAS course online is generally as recognized as one from an offline course. The main factor for recognition is the quality of the curriculum, the industry expert instructors, training, and whether the course prepares you for official Clinical SAS certifications, which are globally recognized irrespective of the training mode.
For clinical trials professionals, the primary and most relevant course is Clinical SAS Certification Course or Advanced Diploma in Clinical SAS offered by Clini Launch. However, you can get prepared for SAS certified Clinical Trials Programmer Using SAS 9.
Obtaining a clinical SAS certification offers you a significant benefit for your career advancements and credibility by:
- Validating your specialized skills in a highly regulated and in-demand niche roles.
- Increasing employability and making a candidate stand out to potential employers in pharmaceutical and contract research organizations (CROs).
- Leading to higher salary packages and better negotiation power.
- Providing a recognized benchmark of proficiency, building trust with employers and colleagues.
- Facilitating faster career progression into senior or lead job roles.
The SAS Clinical Trials Programmer Certification (and most other SAS certifications) typically do not have an expiration date and do not require renewal. Once earned, the certification remains valid. However, given the evolving nature of software and industry standards, SAS often releases updated certification exams reflecting newer versions of their software or industry practices, and professionals may choose to pursue these newer certifications to stay current.
The Clinical SAS Certification exam tests your knowledge in the following key areas:
- Base SAS programming fundamentals (DATA Step PROC Step, functions, arrays).
- Advanced SAS Programming (Macro, SQL, ODS).
- Clinical trials processes and data flow.
- CDISC standards (SDTM and ADaM implementations, Define-XML).
- Generating Tables, Listings, and Figures (TLFs) for clinical study reports.
- Data validation and quality control in a clinical context.
- Understanding of regulatory guidelines relevant to clinical data.
A typical clinical SAS programming course curriculum usually covers core modules such as:
- Base SAS: Data step programming, PROC statements, data manipulation.
- Advanced SAS: SAS Macro language, PROC SQL, SAS Output Delivery System
- Clinical Trial Concepts: Introduction to clinical research, clinical trials phases, and regulatory environment (ICH-GCP, FDA).
- CDISC Standards: Detailed study of SDTM (Study Data Tabulation Model) and ADaM (Analysis Data Model) implementation.
- TLF Generation: Creating Tables, Listings, and Figures for clinical study reports.
- Data Validation and Quality Control: Techniques for ensuring data integrity and accuracy.
A clinical SAS programming course ensure compliance with industry standards like CDISC by:
- In-depth training modules on SDTM and ADaM implementation guidelines.
- Hands-on exercises focus on transforming raw clinical data into CDISC- compliant datasets.
- Teaching the creation of Define-XML and other metadata.
- Emphasizing CDISC controlled terminology and its application.
- Incorporating best practices for data standardization and regulatory submission requirements.
A clinical SAS programming course integrates real-world projects and case studies such as:
- End-to-end simulation of a clinical trial, from raw data import to final report generation.
- Projects focused on data cleaning, validation, and discrepancy resolution.
- Assignments on creating SDTM and ADaM datasets from varied raw data structures.
- To develop SAS programs to generate specific Tables, Listings, and Figures (e.g., demographics table, adverse events listing, efficacy plots).
- Case studies that involve complex data merging and manipulation based on study protocols.
To enroll for a clinical SAS programming course, the eligibility criteria for those from non-technical backgrounds, often include:
- A bachelor’s or master’s degree in a life science domain.
- A strong logical and analytical aptitude.
While programming experience is a plus, Clini Launch’s clinical SAS programming course is specifically designed to teach SAS from the basics, making them accessible to those without a programming background, provided they are keen learners.
The typical career path for someone who completes a clinical SAS Course and enters as a fresher often starts as:
- Junior Clinical SAS programmer/Associate Statistical Programmer
- Progresses to Clinical SAS Programmer/Statistical Programmer (with 1-3 years of experience).
- Then to Senior Clinical SAS Programmer/Senior Statistical Programmer (3-6 Years of experience).
- Followed by Lead Clinical SAS Programmer/Principal Programmer (6+ Years of experience)
- And then potentially into management roles like Manager of Statistical Programming or Associate Director.
A clinical SAS course helps students learning data validation techniques and quality control in clinical trials by:
- Teaching them SAS programming techniques to identify data inconsistencies, outliers, and errors.
- Covering validation programs development and data quality checks.
- Asserting the importance of audit trials, comprehensive documentation, and version control of programming processes.
- Offering hands-on experience in data cleaning cycles and resolve data queries based on industry best practices.
The average starting salary for a clinical SAS professional in India after the course completion typically ranges from the ₹3.5 LPA to ₹6 LPA. The salary range may vary based on the organization, city, place, and hiring companies. However, with experience and official certifications, your salary may increase significantly.
Beyond technical programming, a clinical SAS course aims to develop crucial skills such as:
- Analytical and logical thinking: To understand complex data relationships and problem-solving.
- Attention to detail: It is crucial for accuracy in a highly regulated environment.
- Communication Skills: It is to effectively interact with professionals like Biostatisticians, data managers, and medical writers.
- Clinical research processes: You need to understand how to put programming tasks into clinical context.
- Adherence to quality and regulatory compliance.
The size of Clinical SAS classes at Clini Launch ranges around 10 to 25 students. Our Small class Size generally leads to:
- Personalized attention from mentors
- Doubt Clarification sessions with increase in opportunities for individuals
- Hands-on practical learning sessions with one-on-one coding support.
- Better engagement and interaction among students and the instructors.
Following are the common practical exercises and assignments in clinical SAS classes to reinforce learning include:
- Coding challenges based on real-world clinical scenarios.
- Creating specific CDISC SDTM and ADaM datasets from raw data.
- Developing programs to generate Tables, Listings, and Figures (TLFs) as per mock shells.
- Debugging exercises where students identify and fix errors in pre-written SAS code.
- Data validation tasks to ensure data integrity and quality.
- Mini projects involving a complete workflow from data import to final output.
Clinical SAS classes handle the practical aspects of data security and patient privacy by:
- Using anonymized or simulated datasets that do not contain real patient identifiers.
- Educating students on data privacy regulations like HIPAA and GDPR (though not strictly applicable to India, understanding global standards is key).
- Emphasizing the importance of access controls, secure data handling practices, and ethical considerations in clinical research.
- Discussing the validation and audit trails critical for regulatory compliance.
Clinical SAS classes can be both, depending on the program:
- Intensive weekday programs are typically structured with daily classes and assignments, requiring significant time commitment.
- Weekend or part-time programs offer a more spread-out schedule, allowing for self-paced learning alongside other commitments.
- Many online courses inherently offer more self-paced options with recorded lectures and flexible assignment deadlines. It’s crucial to check the specific institute’s teaching methodology.
The main topics covered in the SAS clinical trial certification exam syllabus typically include:
- Clinical trial process and data flow: Understanding the phases, roles, and data types.
- Base SAS programming: Data step, procedures, functions, arrays, I/O.
- SAS Macro Language: Creating and using macros, macro variables, conditional logic.
- SAS SQL: Querying and joining data, creating tables and views.
- CDISC standards: SDTM and ADaM data models, Define-XML, controlled terminology.
- Generating TLFs: Creating and validating tables, listings, and figures for clinical study reports.
- Data validation and quality control: Techniques and regulatory aspects.
The typical study duration and resource commitment required to pass the SAS clinical trial certification can vary, but generally:
- Duration: 3-6 months of dedicated study after completing a comprehensive training course.
- Resources: Official SAS study guides, practice exams, access to SAS software for hands-on practice, and potentially supplementary books or online tutorials.
- Commitment: Consistent daily or weekly study, focusing heavily on hands-on coding and understanding regulatory context.
The SAS clinical trial certification primarily focuses on the practical application of SAS in clinical settings, though a strong theoretical understanding of clinical trial processes and regulatory guidelines is also crucial. The exam includes scenario-based questions that require candidates to apply their SAS programming skills to solve real-world clinical data challenges, emphasizing data manipulation, analysis, and reporting in a compliant manner.
Yes, there are specific prerequisites or recommended prior SAS certifications before attempting the SAS clinical trial certification. Clini Launch strongly recommend that candidates hold Certification in Clinical SAS and learn from Advanced Diploma in Clinical SAS. It is to ensure a strong foundation in core SAS programming concepts that is essential for clinical trial programming purposes.
The current demand for clinical SAS professionals in the pharmaceutical and clinical research industry in India is consistently high and growing. India is a major hub for Clinical trials and Contract Research Organization activities. It leads to a continuous need for skilled Clinical SAS Programming Professional who can handle and analyse complex clinical data for regulatory submissions and drug development options.
Yes, comprehensive Clinical SAS training at Clini Launch typically includes modules on ethical considerations and data privacy regulations in clinical research. This covers topics like:
- ICH-GCP principles: Ensuring ethical conduct of clinical trials.
- Data privacy laws: Understanding regulations like HIPAA (globally) and equivalent Indian guidelines for handling patient sensitive data.
- Data anonymization and de-identification techniques.
- The importance of data integrity, confidentiality, and security in programming practices.
Clinical SAS training contributes to faster entry into the pharmaceutical or CRO industry for freshers by:
- Providing specialized skills directly aligned with industry demand.
- Equipping them with practical knowledge of CDISC standards and regulatory requirements, which are critical for immediate productivity.
- Often including placement assistance and industry connections.
- Building a strong foundation in statistical programming within a clinical context, making them job ready.
For a Clinical SAS professional, typical career progression opportunities with increasing experience include:
- Senior Clinical SAS Programmer: Taking on more complex studies, mentoring juniors.
- Lead Clinical SAS Programmer: Leading programming activities for entire studies or programs.
- Principal Clinical SAS Programmer: Senior technical expert, often involved in strategic planning and process improvement.
- Manager/Director of Statistical Programming: Overseeing programming teams, resource management, and departmental strategy.
- Biostatistician/Statistical Analyst: For those with strong statistical background and desire for deeper analytical roles.
A Clinical SAS programmer training program aims to instil specific technical skills such as:
- Proficiency in SAS DATA step programming for data manipulation and transformation.
- Expertise in SAS Procedures (e.g., PROC MEANS, PROC FREQ, PROC SQL, PROC REPORT).
- Ability to use the SAS Macro Language for efficient and reusable code.
- Understanding and implementation of CDISC SDTM and ADaM datasets.
- Skills in generating Tables, Listings, and Figures (TLFs) for clinical study reports.
- Knowledge of data validation, quality control, and debugging techniques.
Yes, Clinical SAS programmer training typically covers the use of SAS/GRAPH and ODS (Output Delivery System) for report generation. ODS is crucial for producing high-quality, submission-ready outputs in various formats (RTF, PDF, HTML), while SAS/GRAPH is used for creating complex and customized visualizations (graphs and charts) required for clinical study reports.
Clinical SAS programmer training prepares students and professionals with the job roles involves:
- Writing and validating SAS programs to create datasets (SDTM, ADaM) from raw clinical data.
- Generating Tables, Listings, and Figures (TLFs) based on statistical analysis plans.
- Extensive data quality checks and data inconsistencies to check its performance and resolution.
- Collaboration with professional Biostatisticians, Data Managers, and Medical Writers.
- Maintain Programming code and process documentation.
- Compliance with regulatory guidelines and internal standards.
With the challenge in management and analysis of real-world messy clinical data, a clinical SAS programmer training from CliniLaunch:
- Provides hands-on practical training with anonymized messy datasets to mimic real-world challenges.
- Offer techniques for data cleaning, imputation of missing values, and error handling.
- Highlighting trials programming practices to identify and address data quality issues.
- Training on data validation rules and cross-checking procedures that ensures accuracy.
- To develop problem-solving skills and transform complex data into analysis ready formats.
A top Clinical SAS institute is distinguished by its industry experts and curriculum designed with market relevancy through:
- Industry instructors or former clinical SAS programmers or Statistical programmers with significant industry experience in pharmaceutical companies or Contract Research Organizations.
- Clini Launch faculty members often hold certifications while demonstrating their expertise in Clinical SAS.
- Industry-led expert trainers possess a deep understanding of clinical trials, regulatory guidelines (ICH-GCP, CDISC), and statistical concepts.
- We, at Clini Launch, maintain strong tier with companies while facilitating guest lectures, workshops, and placement opportunities.
Clini Launch- Clinical SAS Institute provides ongoing placement support to its alumni by:
- Maintaining an alumni network for professional connections and job referrals.
- We offer refresher courses on new industry updates or advanced topics.
- Providing continued access to online learning platforms or study materials for one month after the course completion.
- We help alumni with career counselling sessions and mentorship for your career progression.
- We share job alerts and recruitment opportunities within the alumni community.
From a quality Clinical SAS institute, you can expect:
- Modern Computer labs with up-to-date hardware and software.
- Licensed SAS software access (either on-site or cloud-based)
- Comfortable and conducive classrooms for offline learning sessions.
- Gain access to comprehensive study materials and recorded sessions including presentation slides, practical guides and refence documents.
- A supportive learning environment through LMS (Learning Management System Software) with accessible instructors and peer interaction.
Yes, Clini Launch – A clinical SAS institute offers flexible learning options beyond traditional classroom settings to cater to diverse student’s needs:
- Live training sessions with real-time interactions.
- Pre-recorded lectures or training sessions and materials accessible on demand.
- A combination of online and in-person sessions.
- Clini Launch also offers weekly batches for working professionals.
With these options, we provide convenience and accessibility without compromising on the education and training quality.
The key difference between a general SAS programming course and a specialized clinical SAS certification course are:
- General SAS certification course specifically focuses on broad data manipulation and analysis. Clinical SAS specifically targets applications in regulated Clinical Trials.
- Clinical SAS delves deep into CDISC standards (SDTM, ADaM), regulatory guidelines (ICH-GCP, FDA), and clinical trial Processes.
- Clini Launch’s Advanced Diploma in Clinical SAS course aims to produce professionals capable of generating regulatory-compliant outputs (TLFs, Define-XML) for drug development.
- Clinical SAS certification courses specifically prepare for the SAS certified Clinical Trials programmer exam, unlike general SAS courses.
A clinical SAS certification course at Clini Launch validates a candidate’s readiness for industry-specific roles by:
- Providing hands-on practical training with clinical trial datasets and real-world scenarios.
- Spotting regulatory compliance and industry standards like CDISC and GCP.
- Incorporating mock interviews and resume preparation tailored to clinical SAS positions for your career growth and expansion.
- Preparing you for the official SAS clinical Trials programmer certification as a recognized benchmark.
From Clinical SAS certification Course, students can expect post-course career guidance such as:
- Resume and cover letter review
- Mock Interview Sessions and refining
- Guidance on Job search strategies and identification of suitable opportunities
- Networking opportunities with Industry leaders through guest lectures, alumni, and industry professionals.
- Further advice on skill development and career progression paths within the clinical research domain.
While the primary recognition comes from SAS institute itself through its official certification, Clini Launch’s Clinical SAS certification course like Certification in Clincial SAS and Advanced Diploma in Clinical SAS might be recognized or designed in alignment with guidelines from:
- CDISC (Clinical Data Interchange Standards Consortium), whose standards are central to the curriculum.
- Clini Launch holds LSSSDC (Life Sciences Sector Skill Development Council) membership in India with NSDC (National Skill Development Corporation) in India for its healthcare certification, advanced, and post graduated diploma programs.
- IAO (International Accreditation Organization), and IAF (International Accreditation Forum) accreditations indicates our commitment to Industry-relevant and quality training.
A clinical SAS programming online course ensures code validation and debugging skills through:
- Dedicated modules on debugging techniques and common SAS errors.
- Practical exercises that contain errors for students to identify and fix.
- Clini Launch industry expert trainers teach best practices for code documentation and commenting for easier debugging.
- It is useful to provide access to SAS logs and output for error analysis.
- Instructor-led code reviews and personalized feedback on assignments.
- Creating validation programs and checks for data accuracy.
In a clinical SAS programming online course for self-paced learning and review, the resources include:
- Pre-recorded video lectures accessible to you 24/7.
- Comprehensive study materials in PDF or e-book format.
- SAS program code files and sample datasets for practice.
- Quizzes and assessments with instant feedback.
- Access to a Learning Management Systems (LMS) with organized content.
- Discussion forums or communities for peer interaction.
Yes, Clini Launch’s Clinical SAS programming online course – Advanced Diploma in Clinical SAS covers advanced topics include SAS Macros for automation and efficiency. Macro programming is specifically crucial in clinical SAS to:
- Create reusable code for repetitive tasks (e.g., generating similar tables for multiple variables).
- Automate report generation.
- Streamline data cleaning and validation process.
- Handle complex conditional logic and parameterization in programs.
A clinical SAS Programming online course prepares students for typical interview questions by:
- Including modules on interview preparation, covering common technical and behavioural questions.
- Conducting mock interviews and providing constructive feedback.
- Offering resume and cover letter building assistance tailored to industry expectations.
- Discussing real-world scenarios and common challenges faced by clinical SAS programmers enabling students to articulate their problem-solving approaches.
- Providing access to a bank of frequently asked interview questions and sample answers.
Yes, Clini Launch’s Clinical SAS online courses are specifically designed to cater to individuals with a non-statistical background, such as life science graduates (Pharmacy, Biotechnology, etc.). These courses often start with fundamental programming concepts, basic statistics, and then gradually introduce the specialized aspects of clinical SAS to ensure that learners form diverse academic backgrounds can grasp the material effectively.
To effectively participate in a clinical SAS online course, software and hardware requirements include:
- A reliable laptop or desktop computer with at least 8 GB RAM, a decent processor (Intel i5 or equivalent), and sufficient storage.
- An updated web browser (Chrome, Firefox) with a stable internet connection, and access to SAS software (provided via cloud-based platforms by the institute or through SAS university edition installation guidance.
- A functional webcam and microphone for interactive online sessions.
While in-person training offers direct networking opportunities with your peers and professionals, a clinical SAS online course compensates through:
- Online discussion forum (Learning Management System) for peer interaction and collaborative problem-solving.
- Live Q&A sessions allow direct interaction with instructors.
- Virtual networking events or guest lectures from industry professionals.
Clini Launch has its alumni groups for continued networking post-course. While different, effective online courses facilitate meaningful interactions.
Yes, Clinical SAS online courses, particularly those offered by private training providers offer flexible payment plans or instalment options. This is a common feature to make the training more financially accessible to a wider range of students, including working professionals. It is always best to directly inquire with the specific institute about their payment policies.
Clini Launch recent placed students
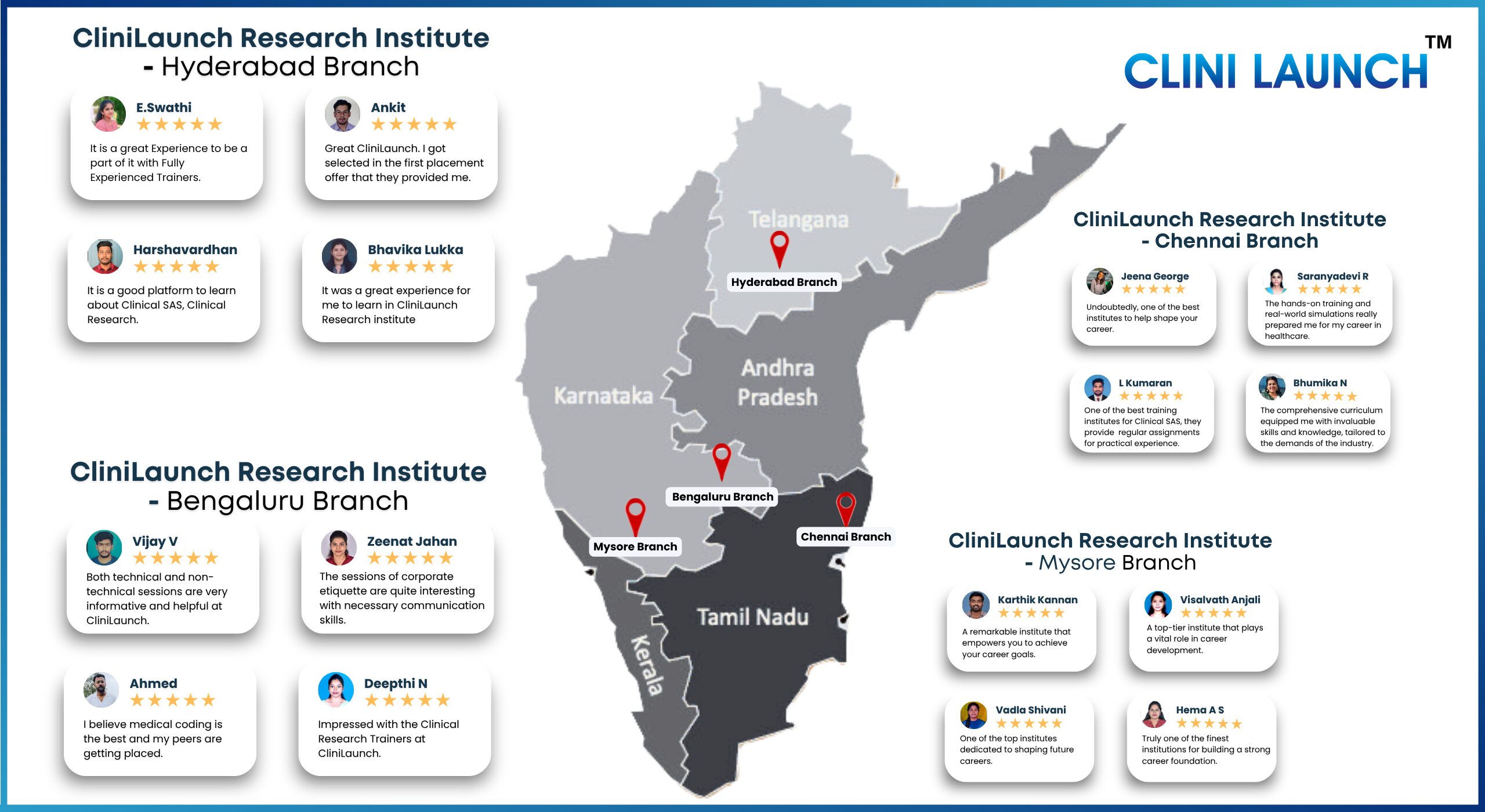











Testimonials
Our upskilling programs make a difference. Hear our graduates speaking for their journey with Clini Launch and how we transformed their career in the healthcare, Information Technology and Pharmaceutical sectors.

